Welcome to the realm of Gizmo Chemical Equations Answer Key, where the intricate world of chemical reactions unfolds with unparalleled clarity. Embark on a journey that unveils the secrets of balanced equations, stoichiometry, and the groundbreaking Gizmo simulation, empowering you to decipher chemical equations with precision and confidence.
Through interactive examples, step-by-step guidance, and real-world applications, this comprehensive resource empowers you to master the art of solving chemical equations, unlocking the mysteries of chemistry and its boundless possibilities.
Chemical Equation Fundamentals: Gizmo Chemical Equations Answer Key
Chemical equations are symbolic representations of chemical reactions. They provide information about the reactants, products, and stoichiometry of a reaction.
A chemical equation consists of the following components:
- Reactants:The substances that are consumed in the reaction.
- Products:The substances that are produced in the reaction.
- Stoichiometry:The coefficients that balance the equation and indicate the relative amounts of reactants and products.
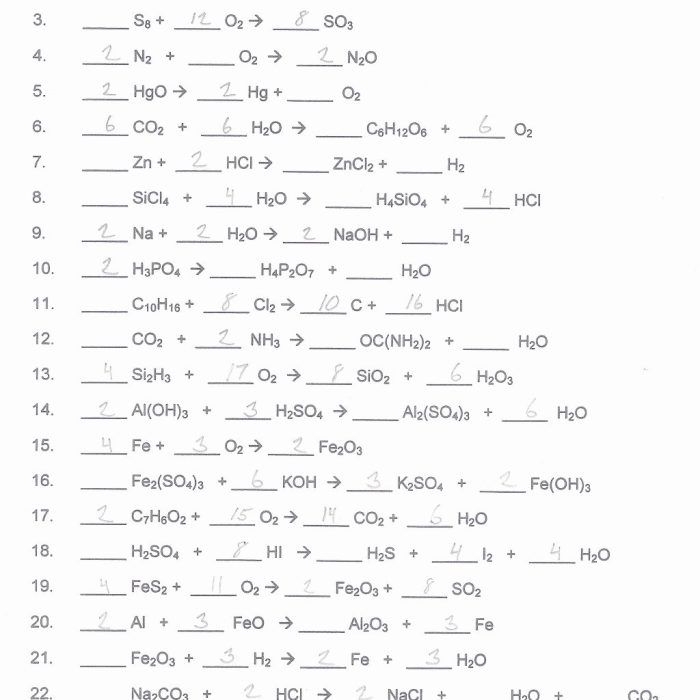
A balanced chemical equation is one in which the number of atoms of each element is the same on both sides of the equation. An unbalanced chemical equation is one in which the number of atoms of each element is not the same on both sides of the equation.
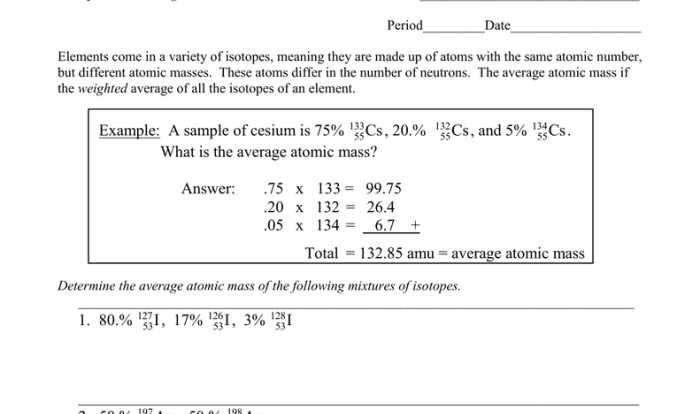
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. Stoichiometry can be used to calculate the amount of reactants or products that are needed or produced in a reaction.
Balancing Chemical Equations
Balancing chemical equations is essential for ensuring that the number of atoms of each element is the same on both sides of the equation. There are several methods for balancing chemical equations, including:
- The half-reaction method:This method involves balancing the oxidation and reduction half-reactions of the reaction separately.
- The oxidation number method:This method involves assigning oxidation numbers to each atom in the reaction and then balancing the equation based on the changes in oxidation numbers.
- The trial and error method:This method involves adjusting the coefficients of the reactants and products until the equation is balanced.
Once a chemical equation is balanced, it can be used to calculate the amount of reactants or products that are needed or produced in a reaction.
Gizmo Chemical Equations Simulation
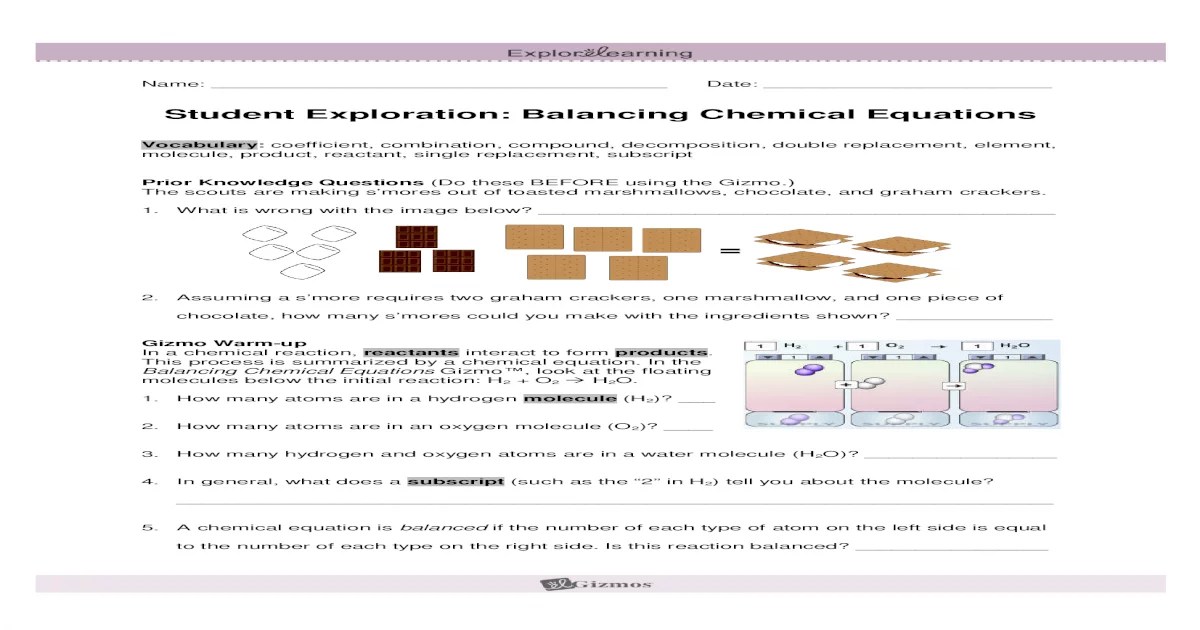
The Gizmo Chemical Equations simulation is an interactive tool that allows students to explore the principles of chemical equations. It provides a visual representation of chemical reactions and helps students understand the relationship between reactants and products.
The simulation has a simple and user-friendly interface. The left side of the screen shows a list of reactants and products. The right side of the screen shows a chemical equation. Students can drag and drop reactants and products into the equation to create different chemical reactions.
Using the Simulation
- To create a chemical reaction, drag and drop reactants from the left side of the screen into the equation on the right side.
- To remove a reactant or product from the equation, drag and drop it back to the left side of the screen.
- To balance a chemical equation, click the “Balance” button. The simulation will automatically adjust the coefficients in the equation to make the number of atoms of each element equal on both sides.
- To see a step-by-step explanation of how the equation was balanced, click the “Show Steps” button.
Tips and Tricks
- Start with simple reactions. As you become more comfortable with the simulation, you can try more complex reactions.
- Use the “Show Steps” button to see how the equation was balanced. This can help you understand the principles of balancing chemical equations.
- Don’t be afraid to experiment. The simulation is a safe environment to try different reactions and see what happens.
Solving Chemical Equations with Gizmo
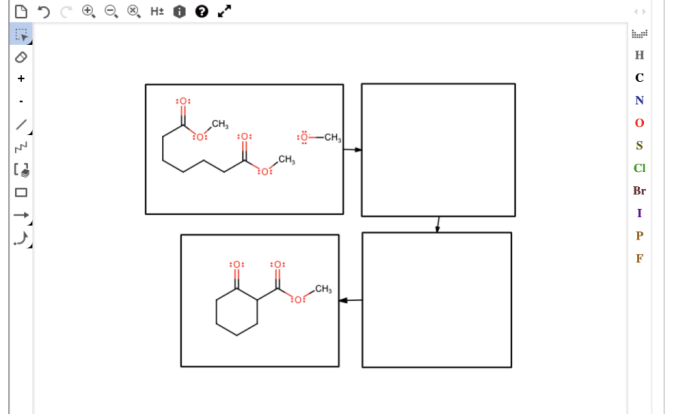
Gizmo’s Chemical Equations simulation provides an interactive platform to solve chemical equations, facilitating a step-by-step approach to balancing and understanding chemical reactions.
Step-by-Step Process
- Enter the unbalanced equation:Input the chemical equation in the Gizmo interface, representing the reactants and products involved in the reaction.
- Adjust coefficients:Use the sliders to adjust the stoichiometric coefficients in front of each chemical formula. These coefficients represent the number of molecules or moles of each substance involved.
- Balance the equation:Drag the coefficients until the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. Gizmo provides real-time feedback, highlighting imbalances and guiding the balancing process.
- Check for balance:Once all elements are balanced, the equation is considered balanced. Gizmo displays a green checkmark to indicate a balanced equation.
Importance of Balancing
Balancing chemical equations is crucial because it ensures that the law of conservation of mass is upheld. This law states that the total mass of the reactants in a chemical reaction is equal to the total mass of the products.
Balancing the equation guarantees that the number of atoms of each element remains the same throughout the reaction, ensuring the accuracy and validity of the chemical equation.
Real-World Applications of Gizmo
Gizmo is a powerful tool that can be used to solve chemistry problems in real-world scenarios. It can be used to:
- Balance chemical equations
- Predict the products of a chemical reaction
- Calculate the amount of reactants or products needed for a reaction
Research
Gizmo can be used in research to help scientists understand the mechanisms of chemical reactions. For example, it can be used to study the kinetics of a reaction, or to identify the intermediates in a reaction.
Industry
Gizmo can be used in industry to help engineers design chemical processes. For example, it can be used to optimize the yield of a reaction, or to reduce the amount of waste produced.
Education
Gizmo can be used in education to help students learn about chemistry. It can be used to illustrate the concepts of chemical reactions, and to help students develop their problem-solving skills.
Limitations and Biases
Gizmo is a powerful tool, but it does have some limitations. For example, it cannot be used to predict the rate of a reaction, or to account for the effects of temperature and pressure.
Additionally, Gizmo is based on a number of assumptions, which can lead to biases in the results. For example, Gizmo assumes that all reactions are stoichiometric, and that all reactants are completely consumed. In reality, these assumptions are not always true.
Advanced Features and Extensions
Gizmo offers several advanced features to assist in solving complex chemical equations:
- Balancing Redox Reactions:Gizmo simplifies the balancing of redox reactions, which involve electron transfer, by providing a systematic approach and detailed explanations.
- Customizable Coefficients:Users can adjust the stoichiometric coefficients in chemical equations, allowing for the exploration of different reaction pathways and product ratios.
- Reaction Mechanisms:Gizmo enables the visualization and exploration of chemical reaction mechanisms, providing insights into the step-by-step processes involved in chemical transformations.
Using Gizmo to Create and Customize Chemical Equations
Gizmo empowers users to create and customize chemical equations:
- Equation Editor:Gizmo features an equation editor that allows for the creation of new chemical equations or the modification of existing ones.
- Database of Compounds:Gizmo provides a comprehensive database of chemical compounds, enabling users to easily select and add reactants and products to their equations.
- Balancing Tools:Gizmo includes automated balancing tools that can assist users in balancing chemical equations, ensuring their accuracy and stoichiometric correctness.
Examples of Using Gizmo to Explore Chemical Reactions and Mechanisms, Gizmo chemical equations answer key
Gizmo provides numerous opportunities to explore chemical reactions and their mechanisms:
- Investigating Acid-Base Reactions:Gizmo allows users to explore acid-base reactions, including neutralization, precipitation, and gas-forming reactions.
- Modeling Combustion Reactions:Gizmo enables the modeling of combustion reactions, providing insights into the role of oxygen and fuel in these exothermic processes.
- Simulating Redox Reactions:Gizmo facilitates the simulation of redox reactions, allowing users to observe the transfer of electrons and the formation of new compounds.
Key Questions Answered
What is the purpose of the Gizmo Chemical Equations simulation?
Gizmo Chemical Equations is an interactive simulation that helps students visualize and solve chemical equations. It provides a user-friendly interface, allowing students to create and manipulate chemical reactions, balance equations, and explore stoichiometry.
How can I use Gizmo to solve chemical equations?
Gizmo provides a step-by-step process for solving chemical equations. Students can input reactants and products, adjust coefficients, and balance the equation using the simulation’s tools. The simulation provides instant feedback, helping students identify errors and refine their solutions.
What are the benefits of using Gizmo in chemistry education?
Gizmo enhances chemistry learning by making abstract concepts more concrete and engaging. It allows students to experiment with chemical reactions, visualize the process of balancing equations, and develop a deeper understanding of stoichiometry. The simulation also fosters critical thinking, problem-solving skills, and collaboration.