Complete the electrophilic addition mechanism below, a fundamental reaction in organic chemistry, involves the addition of an electrophile to a double or triple bond. This intricate process, characterized by the formation of a carbocation intermediate, holds immense significance in various chemical transformations.
Delving into the intricacies of this mechanism, we will explore the stepwise addition of the electrophile, the formation of the carbocation, and the subsequent nucleophilic attack that ultimately leads to the formation of the final product. By unraveling the intricacies of this reaction, we gain a deeper understanding of its applications in organic synthesis and polymerization.
Electrophilic Addition Mechanism: Complete The Electrophilic Addition Mechanism Below
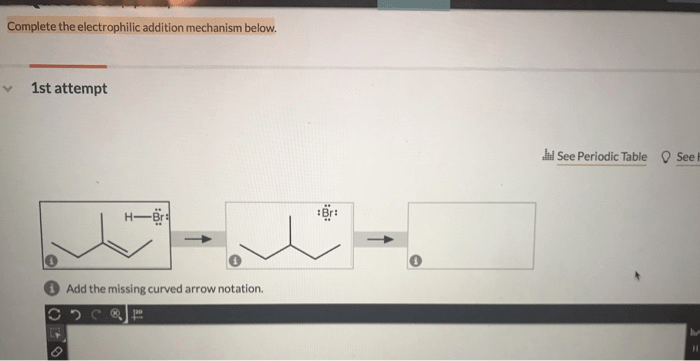
Electrophilic addition reactions are a class of organic reactions in which an electrophile (a species that can accept electrons) adds to a double or triple bond. These reactions are commonly used to synthesize new carbon-carbon bonds and are widely employed in organic chemistry.
The general mechanism of electrophilic addition involves the following steps:
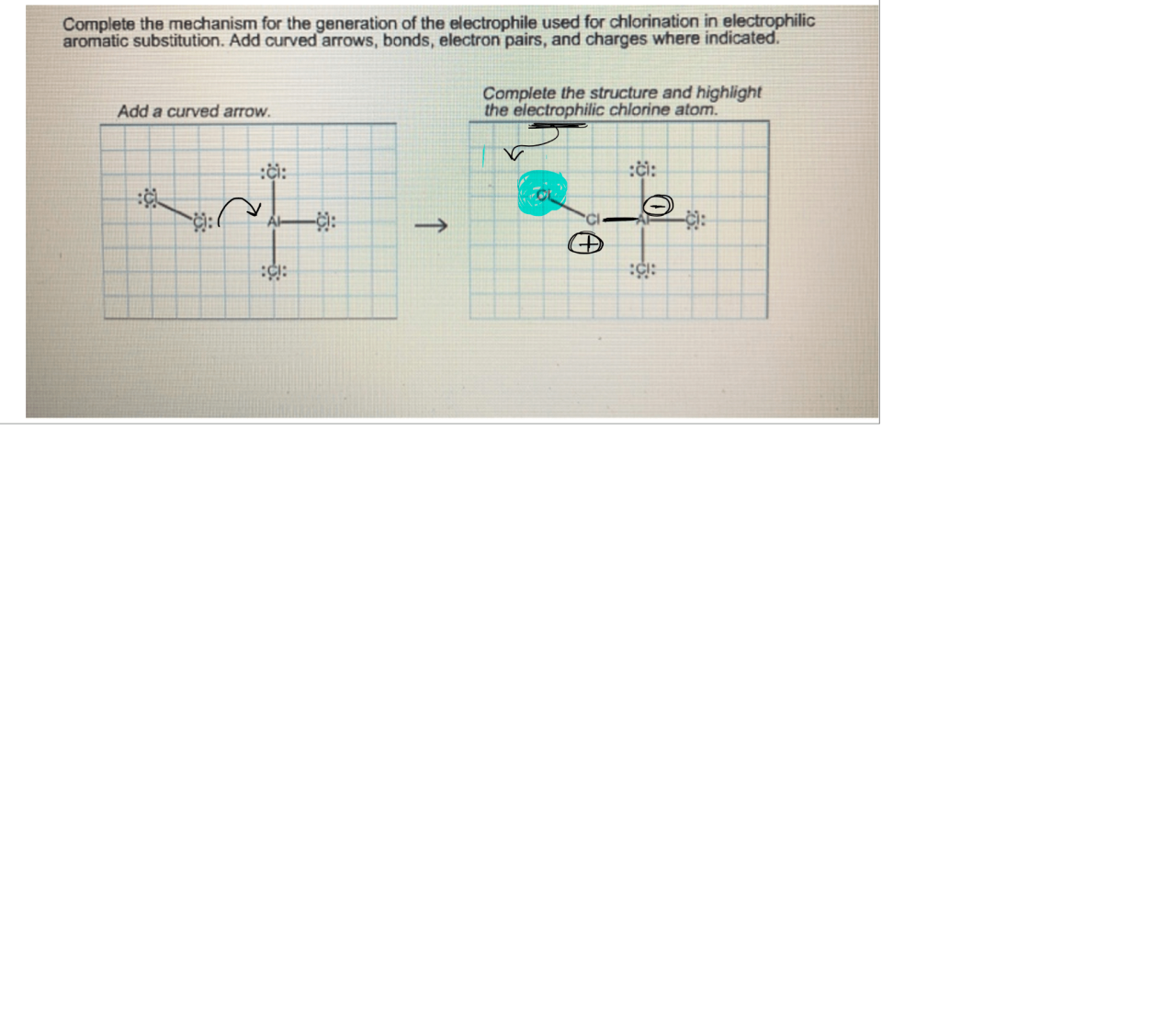
Step 1: Formation of the Electrophile
The electrophile is typically a positively charged ion or a molecule that has an electrophilic functional group. In the case of alkenes and alkynes, the electrophile is formed by the addition of a proton (H +) or a Lewis acid to the double or triple bond.
Step 2: Addition of the Electrophile
The electrophile then adds to the double or triple bond, forming a new carbon-carbon bond. The addition occurs in a concerted manner, meaning that the bond between the electrophile and the carbon-carbon bond is formed simultaneously.
Step 3: Formation of the Carbocation, Complete the electrophilic addition mechanism below
The addition of the electrophile results in the formation of a carbocation, which is a positively charged carbon atom. The stability of the carbocation is crucial for the success of the reaction, as unstable carbocations can undergo rearrangements or other side reactions.
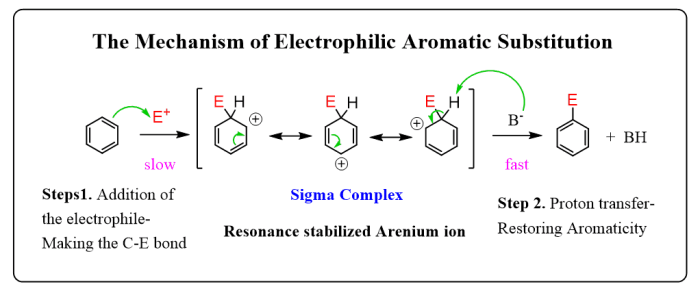
Step 4: Nucleophilic Attack
The carbocation is then attacked by a nucleophile, which is a species that can donate electrons. The nucleophile adds to the carbocation, forming a new bond and neutralizing the positive charge.
Step 5: Formation of the Product
The final step of the electrophilic addition reaction is the formation of the product. The product is typically a substituted alkene or alkyne, depending on the starting material and the nucleophile used.
Question Bank
What is the electrophile in this mechanism?
The electrophile is a species that accepts electrons, typically a positively charged ion or a molecule with an electron-deficient atom.
What is the role of the nucleophile in this mechanism?
The nucleophile is a species that donates electrons, typically a negatively charged ion or a molecule with a lone pair of electrons.