Embark on a scientific expedition as we delve into the captivating realm of average atomic mass, guided by the illuminating Average Atomic Mass Gizmo Answer Key. This comprehensive exploration unravels the intricacies of this fundamental concept, empowering you with a profound understanding of its significance in chemistry.
Through a harmonious blend of theoretical knowledge and practical applications, this guide unveils the secrets of average atomic mass, providing a roadmap for success in your academic pursuits and beyond.
Average Atomic Mass
Average atomic mass, also known as atomic weight, is a weighted average of the masses of all the isotopes of an element. It is a fundamental property of an element that helps determine its chemical and physical properties.
To calculate the average atomic mass, the isotopic masses of each isotope are multiplied by their respective abundances, and the results are summed. The isotopic masses are typically expressed in atomic mass units (amu), and the abundances are expressed as fractions or percentages.
Units of Average Atomic Mass
The units of average atomic mass are atomic mass units (amu). One amu is defined as 1/12th of the mass of a carbon-12 atom. This unit is used because it provides a convenient way to compare the masses of different atoms and elements.
Gizmo Answer Key: Average Atomic Mass Gizmo Answer Key
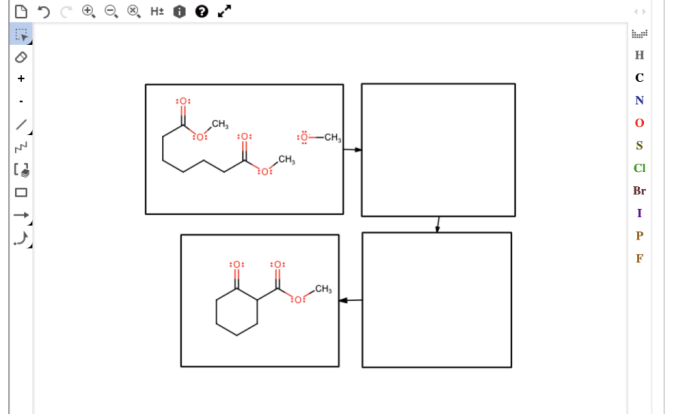
The Gizmo activity on average atomic mass provides an interactive way for students to learn about the concept of average atomic mass. The activity involves using a simulation to create a sample of a compound and then calculating the average atomic mass of the compound.
To complete the Gizmo activity, students will need to follow these steps:
- Select the compound they want to create from the drop-down menu.
- Use the simulation to create a sample of the compound by dragging and dropping atoms into the beaker.
- Calculate the mass of the sample by clicking on the “Calculate Mass” button.
- Calculate the average atomic mass of the compound by dividing the mass of the sample by the number of atoms in the sample.
Here are some tips for using the Gizmo effectively:
- Use the “Show Isotopes” button to see the different isotopes of each element.
- Use the “Show Calculations” button to see the steps involved in calculating the average atomic mass.
- Use the “Reset” button to start over if you make a mistake.
Examples and Applications
Average atomic mass finds numerous applications in chemistry, ranging from determining the properties of elements and compounds to practical applications in various fields.
One crucial application of average atomic mass is in calculating the molar mass of compounds. Molar mass, expressed in grams per mole, represents the mass of one mole of a substance. Knowing the average atomic mass of each element present in a compound allows chemists to determine its molar mass accurately.
Applications in Industry, Average atomic mass gizmo answer key
- Pharmaceuticals:Average atomic mass is used to determine the correct dosage of drugs and medications, ensuring the precise delivery of active ingredients.
- Materials Science:In metallurgy and alloy development, average atomic mass plays a vital role in determining the composition and properties of various alloys.
- Environmental Science:Average atomic mass is used in isotopic analysis to trace the origin and movement of pollutants in the environment.
Applications in Research
- Nuclear Chemistry:Average atomic mass is essential in calculating the mass of atomic nuclei and understanding nuclear reactions.
- Cosmochemistry:By analyzing the average atomic mass of elements in celestial bodies, scientists gain insights into the formation and evolution of stars and planets.
- Geochemistry:Average atomic mass helps determine the composition and age of rocks and minerals, providing valuable information for geological studies.
In summary, average atomic mass is a fundamental concept in chemistry with diverse applications across various fields. It enables precise calculations, accurate predictions, and a deeper understanding of the properties and behavior of elements and compounds.
HTML Table Tags
HTML table tags provide a structured way to organize and present data in a web page. They are especially useful for displaying data in rows and columns, making it easy for users to read and understand.
Creating an HTML Table
To create an HTML table, use the
| tag. For example:
“`html
“` Formatting an HTML TableTo make an HTML table responsive and easy to read, you can use CSS. For example, you can set the width of the table, the height of the rows, and the alignment of the text. Here is an example of CSS that you can use to format an HTML table: “`csstable width: 100%; border-collapse: collapse;th, td border: 1px solid black; padding: 5px;th text-align: center;“` Expert AnswersWhat is the significance of average atomic mass? Average atomic mass plays a pivotal role in determining the properties of elements and compounds, influencing their reactivity, bonding behavior, and overall characteristics. How is average atomic mass calculated? Average atomic mass is calculated by multiplying the isotopic mass of each isotope by its natural abundance and summing the products. What are the units of average atomic mass? Average atomic mass is typically expressed in atomic mass units (amu), which are defined relative to the mass of carbon-12. |